Subretinal Gene Therapy Drug AGTC-501 for X-Linked Retinitis Pigmentosa Phase 2 Randomized, Controlled, Multicenter Clinical Trial (Skyline) 3-Month Results

Andreas K. Lauer, MD Chair, Department of Ophthalmology Thiele-Petti Endowed Chair and Professor Casey Eye Institute – Oregon Health & Science University Portland, Oregon, United States FLORetina ICOOR, Rome, 02 December 2023
Disclosures:
- Ascidian (C)
- Atsena (C)
- Beyeonics (C)
- Beacon (C)
- Blue Rock (C)
- Biogen (C)
- California Institute of Regenerative Medicine Cambridge Consulting (C)
- Editas Genentech Ivericbio (C)
- Johnson & Johnson (C)
- Oxford BioMedica REGENXBIO (C)
- Sanofi TeamedOn (C)
- Vanotech/ORIGEN (C)

RPB Unrestricted GrantNational Institute of Health P30 EY010572Malcom M. Marquis, MD Endowed Fund for Innovation
Co-authors:
- Paul Yang, MD, PhD – Oregon Health Sciences University, Portland Oregon
- David Birch, PhD and Rajiv Anand, MD – Texas Retina Associates, Dallas, Texas
- Robert Sisk, MD – Cincinnati Eye Institute, Cincinnati, Ohio
- Aleksandra Rachitskaya, MD – Cleveland Eye Institute , Cleveland Ohio
- Efren Gonzales, MD – Boston Children’s Hospital, Boston MA
- Sandeep Grover, MD – University of Florida, Jacksonville, Florida
- Nadia Waheed, MD, MPH and Hyung Woo-Kim, PhD – Beacon Therapeutics
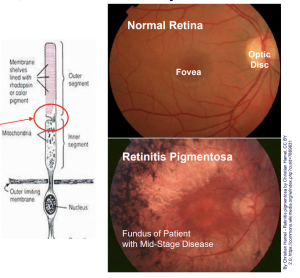
X-Linked Retinitis Pigmentosa: Progressive photoreceptor degeneration that leads to blindness; No treatment options
Severe, aggressive, inherited retinal disease characterized by progressive photoreceptor degeneration
Orphan Disease @ 1:40,000 affecting young males 17K patients in U.S./EU51
Caused by mutation in RPGRorf15 gene, that is responsible for long term maintenance of photoreceptor viability
RPGR localized in cilium, the connective body between inner and outer segment of photoreceptors
Disease progression
- Early – Night blindness
- Mid – Peripheral vision loss
- Late – Central vision loss
Legally blind by median age of 452
2.Vinikoor-Imler LC, et al. Ophthalmic Genet. 2022 Oct;43(5):581-588 2. Chivers M, et al. Clinicoecon Outcomes Res. 2021;13:565–572
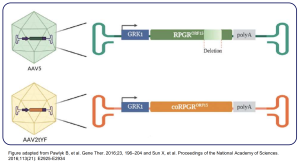
AGTC-501 targets XLRP
- Delivering a correct copy of RPGR gene using AAV vector
- Delivered sub-retinally • Proprietary capsid AAV2tYF
- Photoreceptor specific GRK1 promoter
- Codon optimised to allow for production of fulllength RPGR transgene
- Phase I/II dose open label dose escalation study complete n=29
- Phase II High/Low dose study complete N=14 demonstrating robust improvement in retinal sensitivity

Potential Therapeutic Benefits of Using Full-length RPGR
AGTC-501 is the only late-stage program expected to restore the natural function of photoreceptors
Beacon uses a stable, full-length RPGRORF15 gene therapy vector, overcoming the pitfalls of a truncated RPGRORF15
As a full-length RPGR gene therapy, AGTC-501 therefore has a higher probability of restoring the natural function of cone photoreceptors, yielding greater visual improvement1,2
AGTC-501 and BIIB112 (Biogen) express the same correct full-length RPGR protein and undergo full glutamylation during post-translational modification.
1. Pawlyk B, et al. Gene Ther. 2016;23, 196–204; 2. Sun X, et al. Proceedings of the National Academy of Sciences. 2016;113(21): E2925-E2934
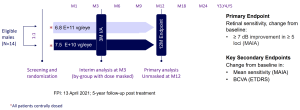
Phase 2 SKYLINE Study Design
Randomized, Controlled, Multicenter Study to Evaluate the Safety and Efficacy of AGTC-501 in Patients with XLRP caused by RPGR mutations
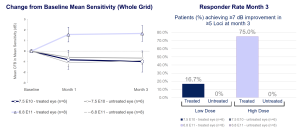
Efficacy Summary for 3-month analysis
Significant improvement in retinal sensitivity demonstrated in the high dose group
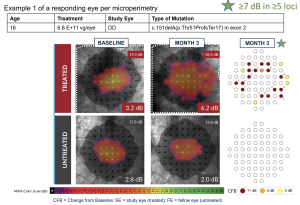
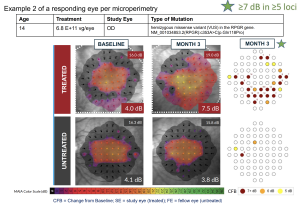
Safety Summary for Month 3 Analysis
No clinically significant safety events related to the study agent
- No Suspected Unexpected Serious Adverse Reactions (SUSARs)
- No endophthalmitis reported
- Majority of ocular AEs were non-serious
- Favorable safety data in both dose groups
- No difference between groups
- 2 ocular SAEs were reported; neither related to study agent
- Persistent decreased vision after surgery, deemed related to study injection
- Increased IOP, deemed related to corticosteroids (concomitant medication)
- 1 non-ocular SAE
- Asthma exacerbation
Ocular SAEs – None study agent-related
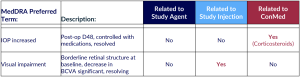
Non-Serious Ocular AEs – Related to Study Agent & All Grade 2 and Transient

Conclusions: AGTC-501 Phase 2 Skyline XLRP 3 Month Interim Results
- Robust and statistically significant improvement in retinal sensitivity in the high dose group
- Response rate of 75% in the high dose (6.8 E+11 vg/eye) group (6/8) at 3 months
- Pattern of response implies therapy rescues photoreceptor sensitivity
- Generally safe and well tolerated
- No clinically significant safety findings related to study agent
- No Suspected Unexpected Serious Adverse Reactions (SUSARs), no endophthalmitis reported
- 2 ocular SAEs were reported; neither related to study agent
- 12 month confirms 3 month data, will be presented at upcoming meeting
Acknowledgments
We would like to thank the Investigators, sites, and study participants and their families who participated in this study.
Funding: Clinical trial sponsored and funded by Beacon Therapeutics. Presenter’s work was supported by the National Institutes of Health (Bethesda, MD) P30 EY010572 core grant and an unrestricted grant from Research to Prevent Blindness (New York, NY) to Casey Eye Institute, Oregon Health & Science University
Medical writing support was provided by Susan Schneider, MD, and Sara Butterworth Connell, OD, consultants to Beacon Therapeutics


